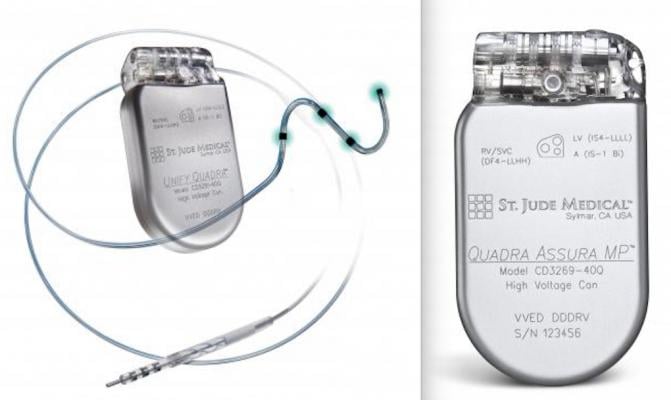
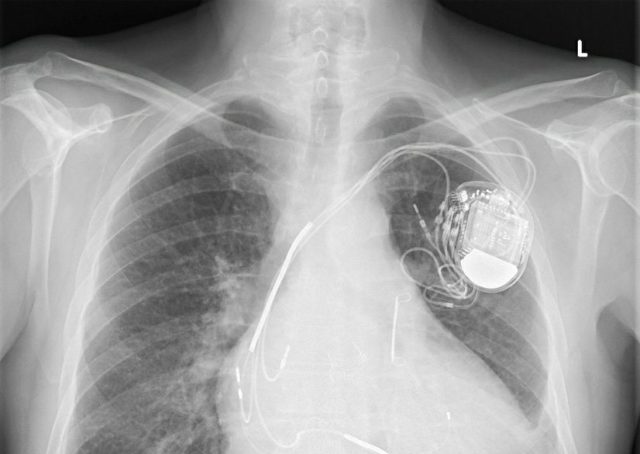
Abbott Releases Planned Security And Performance Updates For Implanted Cardiac Devices | Medical Product Outsourcing

Implantable Defibrillator CD2357-40C, Fortify Assura DR 40, Dual-chamber ICD with RF telemetry, Parylene coating

FDA Approves St. Jude's Ellipse™ and SJM Assura™ Portfolio of ICDs and CRT-D Devices – The World of Implantable Devices
Health Management and Leadership Portal | Implantable cardiac stimulator / cardioverter-defibrillator / automatic Fortify Assura™ ICD St. Jude Medical | HealthManagement.org